We are experts in drug solubility-dissolution studies in presence of surfactants.
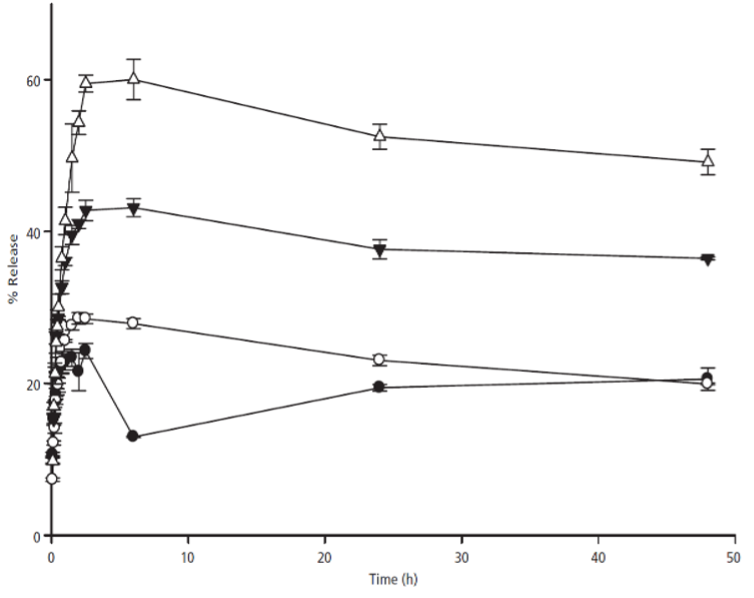
Dissolution profile of Tegretol® tablets in the absence (●) and presence of 0.5 (o), 2 (▼) and 4 (△) mM of TPGS at 10°C .
Charkoftaki G, Dokoumetzidis A, Valsami G, Macheras P. Supersaturated dissolution data and their interpretation: the TPGS-carbamazepine model case.
J Pharm Pharmacol. 2011 Mar;63(3):352-61. doi: 10.1111/j.2042-7158.2010.01226.x. PMID: 21749382.
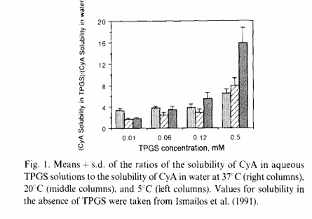
Our group discovered the unusual solubility behavior of cyclosporin (CyA) in 1991
( J Pharm Pharmacol. 1991Apr;43(4):287-9. doi:10.1111/j.20427158.1991.tb06688.x. PMID: 1676746.).
Here, we study the effect of TPCS on CyA solubility:
G. Ismailos, C. Reppas, P. Macheras.Enhancement of cyclosporin
A solubility by d-alphatocopheryl-polyethylene-glycol-1000 succinate (TPGS) European Journal of Pharmaceutical Sciences, 1. 269-271, (1994)
We have explained BCS class migration using a reaction limited dissolution mechanism operating for sparingly soluble drugs
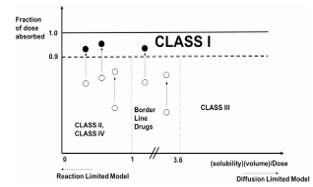
Supersaturation for Class II and IV drugs can lead to class migration (●) or not (○).
Eur J Pharm Sci. 2018 May 30;117:98-106. doi: 10.1016/j.ejps.2018.02.003. Epub 2018 Feb 6. PMID: 29425862.
We developed the reaction limited dissolution model:
The dissolution process is considered as a bidirectional chemical reaction of the undissolved drug species with the free solvent molecules, yielding the dissolved species of drug complex with solvent…The model equation was fitted successfully to dissolution data sets of naproxen and nitrofurantoin formulations measured in the paddle and basket apparatuses, respectively, under various experimental conditions.For comparative purposes these data were also analyzed using three functions based on the diffusion layer model. All functions failed to reveal the governing role of saturation solubility in the dissolution process associated with the diffusion layer model when the conditions for the valid estimation of saturation solubility, established theoretically in this study, were met by the experimental set up employed. (Dokoumetzidis A, Papadopoulou V, Valsami G, Macheras P. Development of a reaction-limited model of dissolution: application to official dissolution tests experiments. Int J Pharm. 2008 May 1;355(1-2):114-25. doi:10.1016/j.ijpharm.2007.11.056. Epub 2007 Dec 5. PMID: 18206324.)
Based on all above, we developed the technique of the in situ preparation of formulation:
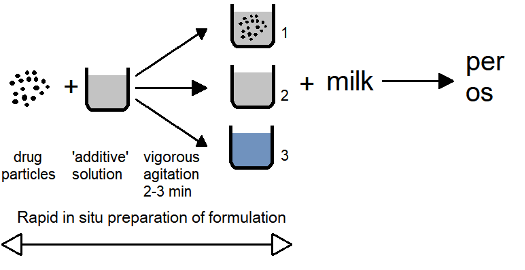
Τhe “additive” is not specified here for reasons of confidentiality. The vigorous agitation accelerates the dissolution-solubilization processes since the initial rate under sink conditions does not depend on the saturation solubility and is proportional to the drug dose and the rate constant controlling the drug-additive surface interaction. A provisional patent has been issued for this technique.
The final dilution of the formulation in milk offers additional solubilization of the undissolved drug species, increased palatability, which can also lead to the development of paediatric formulations. Please note that we are experts in drug-milk based formulations e.g.
Charkoftaki G, Kytariolos J, Macheras P. Novel milk-based oral formulations: proof of concept. Int J Pharm. 2010 May 10;390(2):150-9. doi: 10.1016/j.ijpharm.2010.01.038. Epub 2010 Feb 1. PMID: 20117197.
Soulele K, Karampelas T, Tamvakopoulos C, Macheras P. Enhancement of Docetaxel Absorption Using Ritonavir in an Oral Milk-Based Formulation. Pharm Res. 2021 Aug;38(8):1419-1428. doi: 10.1007/s11095-021-03085-x. Epub 2021 Aug 11. PMID: 34382143.

