We revamped the development of drugs (see https://rdcu.be/epESG)
Early phases and Phase I studies.
Currently, upon completion of the preclinical phase physiologically based pharmacokinetic (PBPK) models, are applied, which are followed by Phase I studies to find the optimal dose and assess safety. For PBPK models focusing on gastrointestinal absorption, the preclinical data of the biopharmaceutical properties solubility, permeability and factors such as dose and drug particle size associated with drug dissolution are routinely used. In the PBPK studies, first-order kinetics is being used since the absorption rate constant ka is linked with the effective permeability Peff and radius R of the gastrointestinal lumen, namely, ka = 2 Peff/R. We have repeatedly questioned the validity of the absorption rate constant ka, and we are now working on a manuscript entitled “PBPK models in oral drug absorption are not optimal: The top down PBFTPK models can better guide oral drug absorption”. We are also aware of the concerns associated with the credibility of PBPK work in oral drug absorption (see Sugano K. Lost in modelling and simulation? ADMET DMPK. 2021 Mar 22;9(2):75-109. doi: 10.5599/admet.923. PMID: 35299768; PMCID: PMC8920108.)
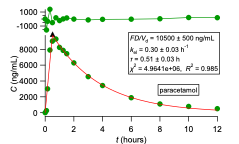
We propose an oral pharmacokinetic study with a small number of subjects; the data are analysed with the top-down PBFTPK models to estimate for the first time absolute bioavailability from oral data exclusively. We simply quote here the fundamental relationship which applies for the simplest model with one input stage with duration τ and one compartment model disposition when absolute bioavailability, is equal to one, F=1.
F=(AUC)_oral/(C(τ) e^(k_el τ) (k_el )^(-1) )+1-(1-e^(-k_el τ))/(k_el τ) (1)
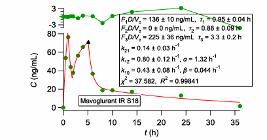
Where is the blood drug concentration at time τ and kel is the elimination rate constant estimate derived from the fitting of the PBFTPK model to the data. (AUC)_Oral is the area under the curve of the oral data calculated with the trapezoidal rule. The complete absorption of drug is justified when the calculation results in F=1. Methodologies for the estimation of F for the different PBFTPK models when F<1 were developed. This speeds up and validates the development process since both the absolute bioavailability and the characteristics of drug absorption are estimated, namely, i) the number of absorption stages, ii) the corresponding drug input rates and iii) the duration of each stage τi, as well as the total duration of absorption, τ.
Phase II and III studies.
The vast majority of published pharmacokinetic, pharmacodynamic and pharmacometrics studies of Phases II and III dealing with oral drug absorption rely on first-order absorption models. In all these studies the fallacious first-order absorption rate constant governs and quantifies the rate of drug absorption. In the field of interspecies scaling and paediatric scaling, the inverse time units of the absorption rate constant do not allow a scaling exercise. In contrast, the physiologically based meaningful input parameters of the PBFTPK models allow the scaling between adult and children or species oral data. Besides, PBFTK models have been applied successfully to the analysis of data currently analyzed with the unphysical flip-flop kinetics and stochastic approaches. Most importantly, we also use PBFTPK models as structural models in population analyses.
Overall, we perform PK, PK-PD classical and populations studies and analyze them with the physiologically sound PBFTPK models.