We write biowaiver reports. We have published relevant work, which can be used in “difficult” cases e.g.
Rinaki E, Dokoumetzidis A, Valsami G, Macheras P. Identification of biowaivers among Class II drugs: theoretical justification and practical examples. Pharm Res. 2004 Sep;21(9):1567-72. doi: 10.1023/b:pham.0000041450.25106.c8. PMID: 15497681.
Daousani C, Macheras P. Scientific considerations concerning the EMA change in the definition of “dose” of the BCS-based biowaiver guideline and implications for bioequivalence. Int J Pharm. 2015 Jan 30;478(2):606-9. doi: 10.1016/j.ijpharm.2014.11.062. Epub 2014 Nov 28. PMID: 25437115.
Most importantly, we are interested in failed biowaiver reports. For these cases, we have developed a methodology based on a simple pharmacokinetic study with five subjects, not published as yet, which can be used to justify experimentally the complete (100%) absorption of drug. The methodology is described in our website page “DRUG DEVELOPMENT”. We carry the pharmacokinetic study, analyze the experimental data using PBFTPK models and write the report justifying or not the biowaiver status. We quote two examples of the PBFTPK model fittings to class I drugs,
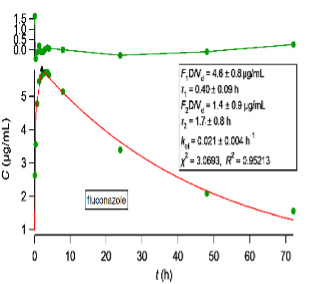
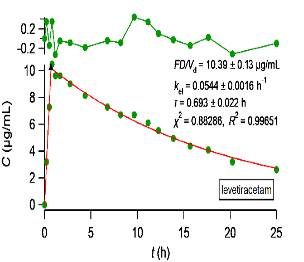
PBFTPK model fits with one (right) or two (left) zero-order input stages and one-compartment model disposition. Optimized parameters are shown in the inset of each graph along with χ2 and R2 for each fit. A solid triangle marks the end of the absorption stage(s). The top panel depicts the fit residuals.

