We have established collaboration with several CROs for the clinical part of the studies. In this context, we perform classical BE studies following the Drug Agencies’ Guidelines. However, we can provide additional services in accordance with client’s tastes, i.e. choosing either 1 or 2 or both or no-one below.
1. Prior to studying.
We have described in the page “DEVELOPMENT OF GENERICS” of our website the methodology for developing IVIVC, if any, for the reference product based on the analysis of the
in vivo data using PBFTPK models. These results guide the dissolution studies with the test product in steps 3 and 4 of the five steps procedure. All results taken together will provide a solid basis for bioequivalence prediction.
It should be noted here that the initial analysis of the in vivo data of the reference product with the PBFTPK models can provide valuable information for the pharmaceutical scientists working on the formulation of generics. See for example our work with carbamazepine (Tegretol) (Alimpertis N, Simitopoulos A, Tsekouras AA, Macheras P. IVIVC Revised. Pharm Res. 2024 Feb;41(2):235-246. doi: 10.1007/s11095-024-03653-x. Epub 2024 Jan 8. PMID: 38191705.) whereas three input stages were found while the duration of absorption was higher than 16h. The current in vitro systems cannot mimic (predict) the complex in vivo absorption profile of carbamazepine while its long duration of absorption rules out the preparation of a sustained release formulation.
2. Upon the completion of the BE study
Apart from the usual BE report we can perform analysis of the data following two methods. The first is a model dependent approach relying on the fitting of the PBFTPK models to the
experimental data of reference and test formulations. The assessment of the extent of absorption is based on the FD/V d estimates, while the assessment of the rate of absorption is based on FD/τV d estimates. The second model independent approach relies on the (AUC) test , (AUC) ref , and (AUC) test /(AUC) ref versus time plots for the assessment of the extent of absorption based on the intersection of the horizontal limb of the curve with the y-axis representing (AUC) test /(AUC) ref . The assessment of the rate of absorption is based on the slope of the cumulative amount of drug absorbed (expressed in terms of concentration) versus time plot. A manuscript entitled “The Finite Absorption Time (F.A.T.) concept reveals a 48-year-long misconception and the need for regulatory bioequivalence guidelines reconsideration” has been submitted for publication. Two are the main conclusions of this work: a. The use of C max , as rate metric in BE studies should be discontinued and b. The assessment of extent of absorption using AUC can be accomplished sooner.
Examples of the two approaches are presented below.
A. Model dependent approach
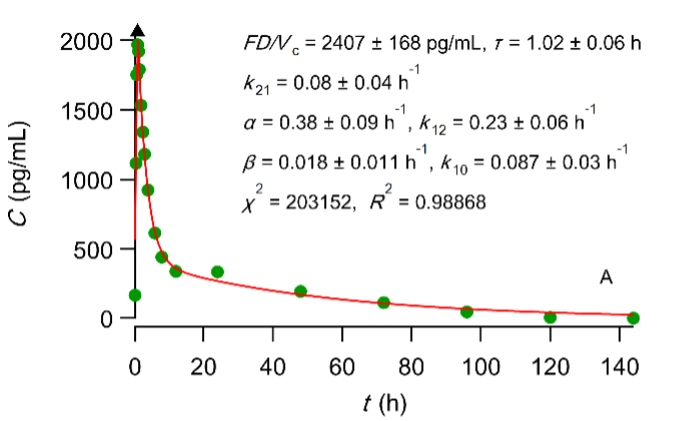
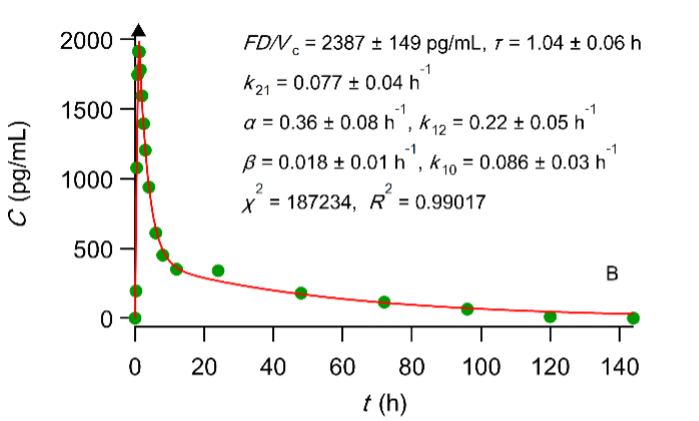
Best fit results of the PBFTPK model with one input stage and two compartment disposition to digoxin experimental test (A) and reference (B) data (see FDA. Center for Drug Evaluation and Research, Digoxin Bioequivalency Review 76268. 2002. ) The symbol ▲ denotes the end of the absorption processes.
A.Model independent approach
i. Extent. The reader can see that the AUC ratio reaches a constant value soon
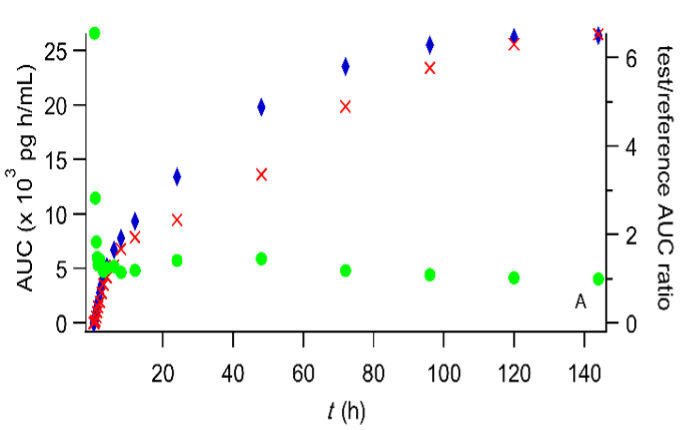

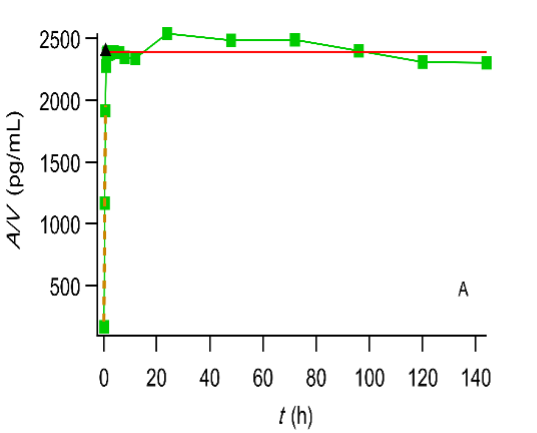
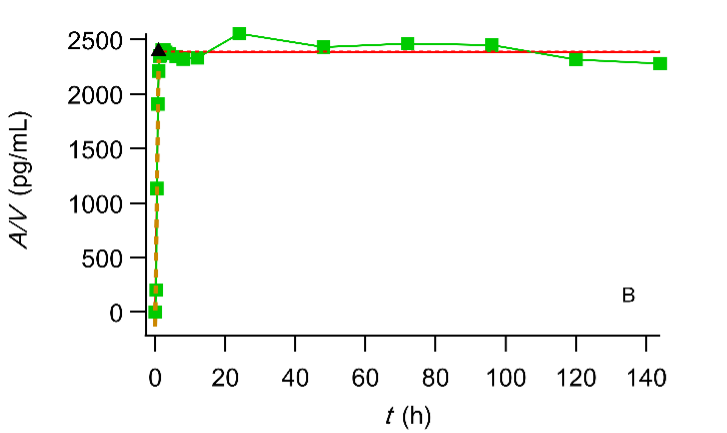
Plot of the cumulative amount of drug absorbed (expressed in terms of concentration) versus time. The symbol ▲ denotes the end of the absorption processes. Digoxin test (A) and reference (B) formulation administered to fasted volunteers (Data reported in (FDA. Center for Drug Evaluation and Research, Digoxin Bioequivalency Review 76268. 2002. https://www.accessdata.fda.gov/drugsatfda_docs/anda/2002/76268_Digoxin_Bioeqr.pdf)
In view of these advances we are very much interested in
A. Rejected bioequivalence studies, and particularly those with confidence interval for Cmax outside the bioequivalence limits (80-125% or 90-111% or wide BE limits for highly variable drugs)
B. Formulations with very rapid absorption e.g. inhalers, whose erratic absorption requires many subjects in order both parameters Cmax and AUC to meet the BE limits criteria. We have proven in our under-review study that the metric is an equally well extent of absorption metric not associated with the early phase data with high variability.
C. Rejected biowaiver reports. Α small bioavailability study analyzed with PBFTPK models demonstrating that absolute bioavailability is equal to one can justify the biowaiver status. See Eq.1 on the DEVELOPMENT OF DRUS page of our website.

