We revamped the development of generics (see https://rdcu.be/epESG)
A PARADIGM SHIFT IN ORAL DRUG ABSORPTION
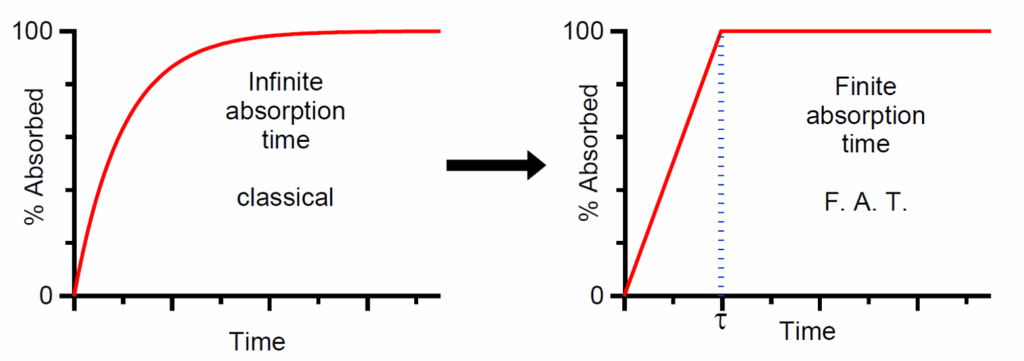
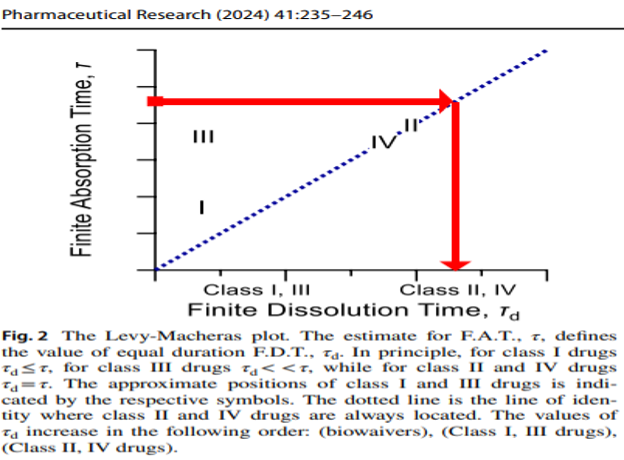
The Wagner-Nelson and Loo-Riegelman methods developed in the 1960s used as such or embedded in deconvolution software for the construction of percent of drug absorbed as a function of time curves were revamped in terms of the physiologically sound F.A.T. concept; the modified %absorbed versus time curves are of bilinear (see scheme above) or multilinear type with the final ascending limb intersecting the horizontal line of 100% drug absorbed at F.A.T. ( doi: 10.1007/s11095-023-03578-x.).The Finite Dissolution Time (F.D.T.) was developed considering the in vivo drug dissolution in terms of the physiological time constraints. Intuitively, for BCS Class II & IV drugs F.A.T.=F.D.T, while for Class I and III drugs F.D.T.< F.A.T. In this context the methodology for IVIVC was revised (doi: 10.1007/s11095-024-03653-x). See relevant published scheme below.
In summary, the steps of our strategy for the development of generics are as follows
1. Analysis of the in vivo reference formulation data using the PBFTPK models
2. Construction, based on the F.A.T. concept, of the percent absorbed versus time plot for the reference formulation
3. In vitro dissolution studies of the reference formulation in a flow through system ensuring sink conditions
4. Correlation based on % absorbed and % dissolved data of the reference formulation considering the F.A.T. and F.D.T. time constraints
5. Dissolution experiments for the generic formulation following steps 3 and 4 above. Compare IVIVC of the two formulations.
In this vein, we are developing a device since all previous dissolution studies focused exclusively on the composition of the dissolution medium, while important aspects of drug dissolution associated with the F.A.T. and F.D.T. concepts have been overlooked for decades. Based on the findings of our work i) drug dissolution/uptake/absorption in the GI tract takes place in a finite time and ii) operate under sink conditions resulting in a linear increase over time of the % absorbed drug; To this end we have designed a biphasic (buffer – n-octanol) dissolution/absorption apparatus based on the F.A.T. principles. This device replicates the dissolution and uptake stages in the stomach and the upper intestine with low pH buffer. We use a blocker as a contact area reducer to simulate transit through the lower intestine with the reduced absorption surface area.

